https://mailchi.mp/79ecc69aca80/the-weekly-gist-december-15-2023?e=d1e747d2d8
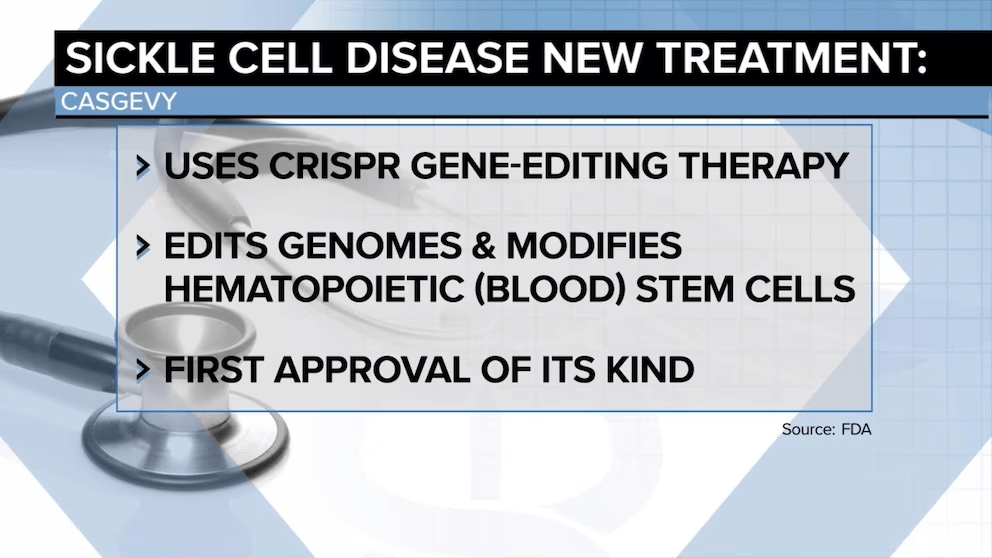
Last week, the Food and Drug Administration (FDA) approved two gene therapy treatments for sickle cell disease, Casgevy and Lyfgenia.
Casgevy, jointly developed by Boston, MA-based Vertex Pharmaceuticals and Switzerland-based CRISPR Therapeutics, is the first approved treatment of any kind available to US patients that uses CRISPR’s gene-editing capabilities.
Lyfgenia, made by Somerville, MA-based Bluebird Bio, uses a more common retrovirus technique for genetic modification. The FDA estimates that about 20K Americans with sickle cell disease will be eligible for the therapies, limited to those patients 12 and older who have had episodes of debilitating pain.
Both treatments will only be available at a small number of facilities nationwide, priced between $2-3M, and require a patient to endure months of hospitalization as well as intensive chemotherapy. Around 100K mostly Black Americans suffer from sickle cell disease, which causes intense pain, organ damage, and reduced life expectancy. Previously, the only curative treatment was a bone marrow transplant.
The Gist: The approval of these drugs represents a milestone moment for those suffering from sickle cell disease, while Casgevy also fulfills the revolutionary promise scientists have seen in CRISPR since it first received broad attention in 2005.
However, now that gene-editing therapies have graduated from the domain of scientific possibility into the realities of our healthcare delivery system, the new challenge becomes ensuring accessibility and equity, as many Americans who most stand to benefit from it also experience barriers in access to care and insurance coverage. (We’d expect insurer pushback similar to that seen when the first highly effective, but extremely costly, hepatitis C treatments like Solvaldi hit the market a decade ago this month.)
While the clinical trial patients who received Casgevy report having “a new lease on life”, sky–high costs, questions of insurance coverage, and the arduous, time-intensive nature of the procedure stand in the way of a population-wide cure for sickle cell disease.