
Cartoon – It’s not the Heat, it’s the Stupidity!





The U.S. may be on the verge of another surge in coronavirus cases, despite weeks of good news.
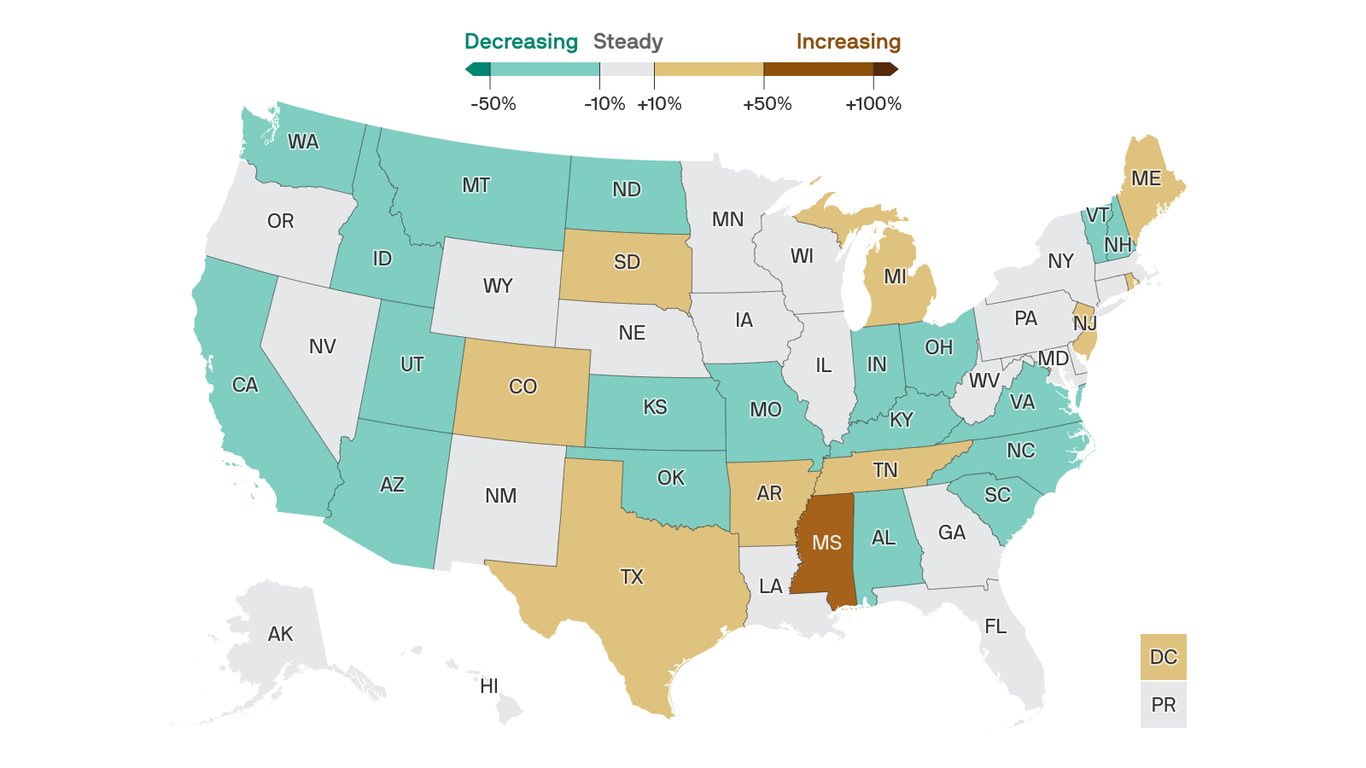
The big picture: Nationwide, progress against the virus has stalled. And some states are ditching their most important public safety measures even as their outbreaks are getting worse.
Where it stands: The U.S. averaged just under 65,000 new cases per day over the past week. That’s essentially unchanged from the week before, ending a six-week streak of double-digit improvements.
What we’re watching: Texas Gov. Greg Abbott on Tuesday rescinded the state’s mask mandate and declared that businesses will be able to operate at full capacity, saying risk-mitigation measures are no longer necessary because of the progress on vaccines.
How it works: If Americans let their guard down too soon, we could experience yet another surge — a fourth wave — before the vaccination campaign has had a chance to do its work.
What’s next: The bigger a foothold those variants can get, the harder it will be to escape COVID-19 — now or in the future.
The bottom line: Variants emerge when viruses spread widely, which is also how people die.

https://mailchi.mp/05e4ff455445/the-weekly-gist-february-26-2021?e=d1e747d2d8

Although the nation reached a grim and long-dreaded milestone on Monday, surpassing 500,000 lives lost to COVID—more than were killed in two World Wars and the Vietnam conflict combined—the news this week was mostly good, as key indicators of the pandemic’s severity continued to rapidly improve.
Over the past two weeks, hospitalizations for COVID were down 30 percent, deaths were down 22 percent, and new cases declined by 32 percent—the lowest levels since late October. This week’s numbers declined somewhat more slowly than last week’s, leading Dr. Rachel Walensky, director of the Centers for Disease Control and Prevention, to caution people against letting their guard down just yet: “Things are tenuous. Now is not the time to relax restrictions.” Of particular concern are new variants of the coronavirus that have emerged in numerous states, including one in New York and another in California, that may be more contagious than the original virus.
The best news of the week was surely a report from the Food and Drug Administration (FDA) evaluating the new, single-shot COVID vaccine from Johnson & Johnson (J&J), showing it to be highly effective at preventing severe disease, hospitalization, and death caused by COVID, including variants. On Friday, a panel of outside experts met to assess whether to approve the J&J vaccine for emergency use, which would make it the third in the nation’s arsenal of COVID vaccines. If approved, the vaccine will be rolled out next week, according to the White House, with up to 4M doses available immediately.
The sooner the better: new data show that since vaccinations began in late December, new cases among nursing home residents have fallen more than 80 percent—a hopeful glimpse at the future that lies ahead for the general population once vaccines become widely available.

Scientists at the Food and Drug Administration said Wednesday that the single-shot Covid-19 vaccine developed by Johnson & Johnson is effective and prevents hospitalizations from the disease.
Johnson & Johnson also revealed new, encouraging data showing the vaccine may do a better-than-expected job at protecting patients against new variants of the virus that causes disease. At the same time, FDA experts said the company’s study, results of which were originally made public in a Jan. 29 press release, includes insufficient information to draw conclusions on efficacy in people older than 75.
Documents from the FDA scientists, as well as separate documents from Johnson & Johnson, were released ahead of a Friday meeting of an FDA advisory panel in which outside experts will discuss and then vote on the risks and benefits of the new vaccine. The panel, known as the Vaccines and Related Biological Products Advisory Committee, makes recommendations to the FDA; the agency is not required to follow them, but it generally does.
The J&J vaccine is the first vaccine to show efficacy given as a single dose. It also does not need to be kept frozen when being shipped, as the vaccines developed by Moderna and the team of Pfizer and BioNTech do. Both of those advantages could be profound when it comes to vaccinating as many people as possible, a key step in slowing the spread of SARS-CoV-2.
Overall in the study, the vaccine reduced cases of Covid-19 that were rated as moderate to severe by 66.1% when considering cases occurring at least 28 days after vaccination. There were 193 cases that occurred at least 28 days after vaccination in the placebo group and 66 in the vaccine group. As of Feb. 5, there were seven Covid-19 related deaths in the placebo group and none in the vaccine group.
FDA researchers conducted a new analysis of how frequently volunteers in the study were hospitalized for Covid. When researchers counted cases 28 days after vaccination, there were zero hospitalizations in the vaccine arm and 16 in the placebo arm. For the full analysis set starting with the first dose, there were six hospitalizations for those who received the vaccine and 42 for those who did not.
Johnson & Johnson and the National Institutes of Health initially announced interim results of a 44,325 study testing the vaccine’s efficacy on Jan. 29. At the time, they said the 66% efficacy varied by geography. The vaccine was 72% protective in the U.S., compared to 58% in South Africa, where a new variant of SARS-CoV-2 is circulating.
In new documents, Johnson & Johnson said that in South Africa, the vaccine reduced severe or critical Covid-19 by 81.7% starting 28 days after vaccination, but that efficacy against more moderate disease was 64%. But the company said that the vaccine efficacy was not affected by the high prevalence of another variant in Brazil.
Unexpected side effects occurred at the same rate overall among volunteers who received vaccine and placebo — about 0.5%. However, some rare conditions appeared more common with the vaccine. Blood clot-related conditions occurred in 15 volunteers who received the vaccine and 10 who received placebo. Tinnitus, a ringing in the ears, occurred in six volunteers who received the vaccine and none who received placebo. The FDA said it will recommend monitoring for thromboembolic events after an EUA is granted.
Expected side effects that are related to the vaccine’s effect were common. Nearly half of volunteers reported injection site pain, 38.9% reported headache, 38.2% fatigue, and 33% reported muscle aches.
Johnson & Johnson also conducted an analysis in 2,650 volunteers looking at whether those who received the vaccine were less likely to test positive for the SARS-CoV-2 virus, which causes Covid-19, without having symptoms. There were 50 such cases in the placebo group compared to 18 among those who received the vaccine, a 65.5% reduction.
The United States has purchased 100 million doses of the vaccine, with an option to buy another 200 million doses. The agreement, announced last August, netted J&J over $1 billion in a contract with the Biomedical Advanced Research and Development Authority and the Department of Defense.
That said, the company currently has a limited number of doses to contribute to the effort to step up the country’s vaccine rollout. It will be April before J&J begins to have substantial amounts of vaccine to feed into the distribution pipeline, Moncef Slaoui, former co-chair of Operation Warp Speed, said earlier this year.
However, the company and the NIH said the vaccine was 85% effective at preventing severe disease, with no differences seen across the eight countries included in the study.
J&J is also conducting a trial in the United States of a two-dose vaccine, with the doses given eight weeks apart.The results from that 30,000 person trial are not expected until sometime in May.
The FDA documents represent the first close look at the data released Jan. 29, and are the result of a three-week effort by FDA scientists to independently evaluate the data generated in the trial. Friday’s panel will provide a deeper look at what those data actually mean.
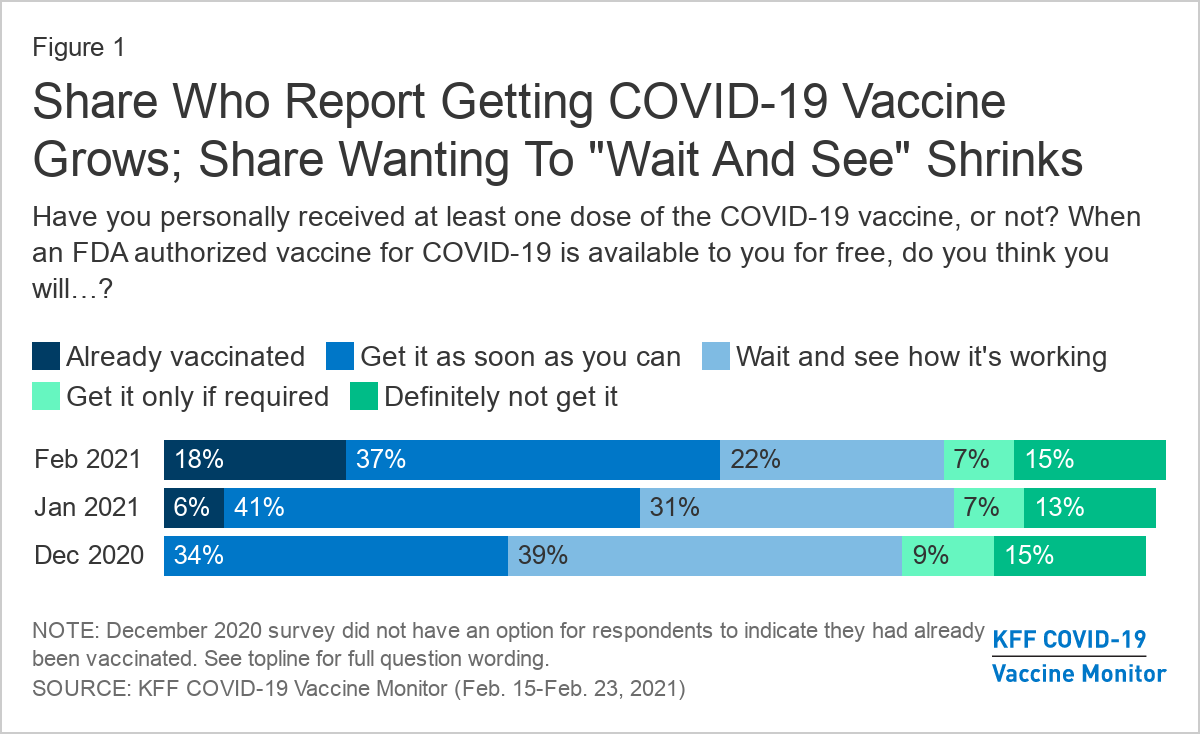
More than half of adults in the U.S. (55%) say they’ve already gotten one dose of Covid-19 vaccine or they’re eager to get one as soon as they can, an increase in acceptance from January (47%), a new poll reports. About 1 in 5 people are waiting to see how the vaccine rollout goes, but don’t rule out vaccination. Another 1 in 5 people are more reluctant: 7% would get vaccinated only if required by work, school, or some other activity, and 15% say no to vaccine under any circumstance. The increase in eagerness spans all demographic groups, but Black adults and young adults under age 30 were most likely to say they want to wait and see.