Aggressive screening might have helped contain the virus in the United States. But technical flaws, regulatory hurdles and lapses in leadership let it spread undetected for weeks.
Early on, the dozen federal officials charged with defending America against the coronavirus gathered day after day in the White House Situation Room, consumed by crises. They grappled with how to evacuate the United States consulate in Wuhan, China, ban Chinese travelers and extract Americans from the Diamond Princess and other cruise ships.
The members of the coronavirus task force typically devoted only five or 10 minutes, often at the end of contentious meetings, to talk about testing, several participants recalled. The Centers for Disease Control and Prevention, its leaders assured the others, had developed a diagnostic model that would be rolled out quickly as a first step.
But as the deadly virus spread from China with ferocity across the United States between late January and early March, large-scale testing of people who might have been infected did not happen — because of technical flaws, regulatory hurdles, business-as-usual bureaucracies and lack of leadership at multiple levels, according to interviews with more than 50 current and former public health officials, administration officials, senior scientists and company executives.
The result was a lost month, when the world’s richest country — armed with some of the most highly trained scientists and infectious disease specialists — squandered its best chance of containing the virus’s spread. Instead, Americans were left largely blind to the scale of a looming public health catastrophe.
The absence of robust screening until it was “far too late” revealed failures across the government, said Dr. Thomas Frieden, the former C.D.C. director. Jennifer Nuzzo, an epidemiologist at Johns Hopkins, said the Trump administration had “incredibly limited” views of the pathogen’s potential impact. Dr. Margaret Hamburg, the former commissioner of the Food and Drug Administration, said the lapse enabled “exponential growth of cases.”
And Dr. Anthony S. Fauci, a top government scientist involved in the fight against the virus, told members of Congress that the early inability to test was “a failing” of the administration’s response to a deadly, global pandemic. “Why,” he asked later in a magazine interview, “were we not able to mobilize on a broader scale?”
Across the government, they said, three agencies responsible for detecting and combating threats like the coronavirus failed to prepare quickly enough. Even as scientists looked at China and sounded alarms, none of the agencies’ directors conveyed the urgency required to spur a no-holds-barred defense.
Dr. Robert R. Redfield, 68, a former military doctor and prominent AIDS researcher who directs the C.D.C., trusted his veteran scientists to create the world’s most precise test for the coronavirus and share it with state laboratories. When flaws in the test became apparent in February, he promised a quick fix, though it took weeks to settle on a solution.
The C.D.C. also tightly restricted who could get tested and was slow to conduct “community-based surveillance,” a standard screening practice to detect the virus’s reach. Had the United States been able to track its earliest movements and identify hidden hot spots, local quarantines might have confined the disease.
Dr. Stephen Hahn, 60, the commissioner of the Food and Drug Administration, enforced regulations that paradoxically made it tougher for hospitals, private clinics and companies to deploy diagnostic tests in an emergency. Other countries that had mobilized businesses were performing tens of thousands of tests daily, compared with fewer than 100 on average in the United States, frustrating local health officials, lawmakers and desperate Americans.
Alex M. Azar II, who led the Department of Health and Human Services, oversaw the two other agencies and coordinated the government’s public health response to the pandemic. While he grew frustrated as public criticism over the testing issues intensified, he was unable to push either agency to speed up or change course.
Mr. Azar, 52, who chaired the coronavirus task force until late February, when Vice President Mike Pence took charge, had been at odds for months with the White House over other issues. The task force’s chief liaison to the president was Mick Mulvaney, the acting White House chief of staff, who was being forced out by Mr. Trump. Without high-level interest — or demands for action — the testing issue festered.
At the start of that crucial lost month, when his government could have rallied, the president was distracted by impeachment and dismissive of the threat to the public’s health or the nation’s economy. By the end of the month, Mr. Trump claimed the virus was about to dissipate in the United States, saying: “It’s going to disappear. One day — it’s like a miracle — it will disappear.”
By early March, after federal officials finally announced changes to expand testing, it was too late. With the early lapses, containment was no longer an option. The tool kit of epidemiology would shift — lockdowns, social disruption, intensive medical treatment — in hopes of mitigating the harm.
Now, the United States has more than 100,000 coronavirus cases, the most of any country in the world. Deaths are rising, cities are shuttered, the economy is sputtering and everyday life is upended. And still, many Americans sickened by the virus cannot get tested.
In a statement, Judd Deere, a White House spokesman, said that “any suggestion that President Trump did not take the threat of Covid-19 seriously or that the United States was not prepared is false.” He added that at Mr. Trump’s direction, the administration had “expanded testing capacities.”
Dr. Bruce Aylward, a senior adviser at the World Health Organization, led an expert team to China last month to research the mysterious new virus. Testing, he said, was “absolutely vital” for understanding how to defeat a disease — what distinguishes it from others, the spectrum of illness and, most important, its path through populations.
“You want to know whether or not you have it,” Dr. Aylward said. “You want to know whether the people around you have it. Because you know what? Then you could stop it.”
“You can’t stop it,” he warned, “if you can’t see it.”
A Startling Setback
The first time Dr. Robert Redfield heard about the severity of the virus from his Chinese counterparts was around New Year’s Day, when he was on vacation with his family. He spent so much time on the phone that they barely saw him. And what he heard rattled him; in one grim conversation about the virus days later, George F. Gao, the director of the Chinese Center for Disease Control and Prevention, burst into tears.
Dr. Redfield, a longtime AIDS researcher, had never run a government agency before his appointment to lead the C.D.C. in 2018. Until then, his biggest priorities had been fighting the opioid epidemic and the spread of H.I.V. Suddenly, a man who preferred treating patients in Haiti or Africa to being in the public glare was facing a new pandemic threat.
At first, Dr. Redfield’s agency moved quickly.
On Jan. 7, the C.D.C. created an “incident management system” for the coronavirus and advised travelers to Wuhan to take precautions. By Jan. 20, just two weeks after Chinese scientists shared the genetic sequence of the virus, the C.D.C. had developed its own test, as usual, and deployed it to detect the country’s first coronavirus case.
“That’s our prime mission,” Dr. Redfield said later in an interview, “to get eyes on this thing.”
Assessing the virus would prove challenging. It was so new that scientists had little information to work with. China provided limited data, and rebuffed an early attempt by Mr. Azar and Dr. Redfield to send C.D.C. experts there to learn more. That the virus could cause no symptoms and still spread — something not initially known — made it all the more difficult to understand.
To identify the virus, the C.D.C. test used three small genetic sequences to match up with portions of a virus’s genome extracted from a swab. A German-developed test that the W.H.O. was distributing to other countries used just two, potentially making it less precise.
But soon after the F.D.A. cleared the C.D.C. to share its test kits with state health department labs, some discovered a problem. The third sequence, or “probe,” gave inconclusive results. While the C.D.C. explored the cause — contamination or a design issue — it told those state labs to stop testing.
The startling setback stalled the C.D.C.’s efforts to track the virus when it mattered most. By mid-February, the nation was testing only about 100 samples per day, according to the C.D.C.’s website.
Dr. Redfield played down the problem in task force meetings and conversations with Mr. Azar, assuring him it would be fixed quickly, several administration officials said.
With capacity so limited, the C.D.C.’s criteria for who was tested remained extremely narrow for weeks to come: only people who had recently traveled to China or had been in contact with someone who had the virus.
The lack of tests in the states also meant local public health officials could not use another essential epidemiological tool: surveillance testing. To see where the virus might be hiding, nasal swab samples from people screened for the common flu would also be checked for the coronavirus.
The C.D.C. announced a plan on Feb. 14 to perform the screening in five high-risk cities: New York, Chicago, Los Angeles, San Francisco and Seattle. An agency official said it could provide “an early warning signal to trigger a change in our response strategy.” But most of the cities could not carry it out.
“Had we had done more testing from the very beginning and caught cases earlier,” said Dr. Nuzzo, of Johns Hopkins, “we would be in a far different place.”
The consequences became clear by the end of February. For the first time, someone with no known exposure to the virus or history of travel tested positive, in the Seattle area, where the U.S.’s first case had been detected more than a month earlier. The virus had probably been spreading there and elsewhere for weeks, researchers later concluded. Without a more complete picture of who had been infected, public health workers could not do “contact tracing” — finding all those with whom any contagious people had interacted and then quarantining them to stop further transmission.
The C.D.C. gave little thought to adopting the test being used by the W.H.O. The C.D.C.’s test was working in its own lab — still processing samples from states — which gave agency officials confidence. Dr. Anne Schuchat, the agency’s principal deputy director, would later say that the C.D.C. did not think “we needed somebody else’s test.”
And the German-designed W.H.O. test had not been through the American regulatory approval process, which would take time.
Throughout February, Dr. Redfield shuttled between Atlanta, where the C.D.C. is based, and Washington, holding multiple calls every day with Mr. Azar and participating in the coronavirus task force.
Mr. Azar’s take-charge style contrasted with the more deliberative manner of Dr. Redfield, who lacked the kind of commanding television presence that impressed Mr. Trump. He was “a consensus person,” as one colleague described him, who sought to avoid conflict. He relied heavily on some of the C.D.C.’s career scientists, like Dr. Schuchat and Dr. Nancy Messonnier, the director of the agency’s National Center for Immunization and Respiratory Diseases.
Under scrutiny from Congress, Dr. Redfield offered reassurances. Responding on Feb. 24 to a letter from 49 members of Congress about the need for testing in the states, he wrote, “CDC’s aggressive response enables us to identify potential cases early and make sure that they are properly handled.”
Days later, his agency provided a workaround, telling state and local health department labs that they could finally begin testing. Rather than awaiting replacements, they should use their C.D.C. test kits and leave out the problematic third probe.
Meanwhile, the agency’s epidemiologists were growing more concerned as the virus spread in South Korea and Italy. On Feb. 25, Dr. Messonnier gave a briefing with a much blunter warning than usual. “Disruption to everyday life might be severe,” she said.
Mr. Trump, returning from a trip to India, was furious, according to senior administration officials. Later that day, Mr. Azar seemed to be tamping down the level of concern. All Dr. Messonnier had meant, he said at a news conference, was that people should “start thinking about, in their own lives, what that might involve.”
“Might,” Mr. Azar repeated emphatically. “Might involve.”
Barriers to Testing
Dr. Stephen Hahn’s first day as F.D.A. commissioner came just six weeks before Mr. Azar declared a public health emergency on Jan. 31. A radiation oncologist and researcher who helped turn around MD Anderson in Houston, one of the nation’s leading cancer centers, Dr. Hahn had come to Washington to oversee a sprawling federal agency that regulates everything from lifesaving therapies to dog food.
But overnight, his mission — to manage 15,000 employees in a culture defined by precision and caution — was upended. A pathogen that Mr. Trump would later call the “invisible enemy” was hurtling toward the United States. It would fall to the newly arrived Dr. Hahn to help build a huge national capacity for testing by academic and private labs.
Instead, under his leadership, the F.D.A. became a significant roadblock, according to current and former officials as well as researchers and doctors at laboratories around the country.
Private-sector tests were supposed to be the next tier after the C.D.C. fulfilled its obligation to jump-start screening at public labs. In other countries hit hard by the coronavirus, governments acted quickly to speed tests to their populations. In South Korea, for example, regulators in early February summoned executives from 20 medical manufacturers, easing rules as they demanded tests.
But Dr. Hahn took a cautious approach. He was not proactive in reaching out to manufacturers, and instead deferred to his scientists, following the F.D.A.’s often cumbersome methods for approving medical screening.
Even the nation’s public health labs were looking for the F.D.A.’s help. “We are now many weeks into the response with still no diagnostic or surveillance test available outside of C.D.C. for the vast majority of our member laboratories,” Scott Becker, chief executive of the Association of Public Health Laboratories, wrote to Mr. Hahn in late February. “We believe a more expeditious route is needed at this time.”
Ironically, it was Mr. Azar’s emergency declaration that established the rules Dr. Hahn insisted on following. Designed to make it easier for drugmakers to pursue vaccines and other therapies during a crisis, such a declaration lets the F.D.A. speed approvals that could otherwise take a year or more.
But the emergency announcement created a new barrier for hospitals and laboratories that wanted to create their own tests to diagnose the coronavirus. Usually, they faced minimal federal regulation. But once Mr. Azar took action, they were subject to an F.D.A. process called an “emergency use authorization.”
Even though researchers around the country quickly began creating tests that could diagnose Covid-19, many said they were hindered by the F.D.A.’s approval process. The new tests sat unused at labs around the country.
Stanford was one of them. Researchers at the world-renowned university had a working test by February, based on protocols published by the W.H.O. The organization had already delivered more than 250,000 of the German-designed tests to 70 laboratories around the world, and doctors at the Stanford lab wanted to be prepared for a pandemic.
“Even if it didn’t come, it would be better to be ready than not to be ready,” said Dr. Benjamin Pinsky, the lab’s medical director.
But in the face of what he called “relatively tight” rules at the F.D.A., Dr. Pinsky and his colleagues decided against even trying to win permission. The Stanford clinical lab would not begin testing coronavirus samples until early March, when Dr. Hahn finally relaxed the rules.
Executives at bioMérieux, a French diagnostics company, had a similar experience. The company makes a countertop testing system, BioFire, that is routinely used to check for the flu and other respiratory illnesses in 1,700 hospitals around the country. It can provide results in about 45 minutes.
“A lot of us said, you know, your typical E.U.A. is just much too demanding,” said Dr. Mark Miller, the company’s chief medical officer, referring to the emergency approval. “It’s going to take much too much time. And can’t you do something to shorten that?”
Officials at the F.D.A. tried to be responsive, Dr. Miller said. But rather than throw out the rules, the agency only modified the regulatory requirements, still requiring weeks of discussions and negotiations.
After conversations with the F.D.A. in mid-February, the company received emergency approval for its BioFire test on March 24. (The company also began talking to the F.D.A. in January about another type of test, but decided not to pursue it in the United States for now.) Dr. Miller said that while he was ultimately satisfied with the F.D.A.’s actions, the overall response by the government was too slow, especially when it came to logistical questions like getting enough testing supplies to those who needed them.
“You’ve got other countries — and I’m sorry, unfortunately, the U.S. is one of those — where they’ve been slow, disorganized,” he said. “There are still not enough tests available there to test everybody who needs it.”
In an emailed statement, Dr. Hahn maintained that his agency had moved as quickly as it safely could to ensure that tests would be accurate. “Since the early days of this pandemic,” he said, “the F.D.A.’s doors have always been and still remain open to test developers.”
A Lack of Trust
Alex Azar had sounded confident at the end of January. At a news conference in the hulking H.H.S. headquarters in Washington, he said he had the government’s response to the new coronavirus under control, pointing out high-ranking jobs he had held in the department during the 2003 SARS outbreak and other infectious threats.
“I know this playbook well,” he told reporters.
A Yale-trained lawyer who once served as the top attorney at the health department, Mr. Azar had spent a decade as a top executive at Eli Lilly, one of the world’s largest drug companies. But he caught Mr. Trump’s attention in part because of other credentials: After law school, Mr. Azar was a clerk for some of the nation’s most conservative judges, including Justice Antonin Scalia of the Supreme Court. And for two years, he worked as Ken Starr’s deputy on the Clinton Whitewater investigation.
As Mr. Trump’s second health secretary, confirmed at the beginning of 2018, Mr. Azar has been quick to compliment the president and focus on the issues he cares about: lowering drug prices and fighting opioid addiction. On Feb. 6 — even as the W.H.O. announced that there were more than 28,000 coronavirus cases around the globe — Mr. Azar was in the second row in the White House’s East Room, demonstrating his loyalty to the president as Mr. Trump claimed vindication from his impeachment acquittal the day before and lashed out at “evil” lawmakers and the F.B.I.’s “top scum.”
As public attention on the virus threat intensified in January and February, Mr. Azar grew increasingly frustrated about the harsh spotlight on his department and the leaders of agencies who reported to him, according to people familiar with the response to the virus inside the agencies.
Described as a prickly boss by some administration officials, Mr. Azar has had a longstanding feud with Seema Verma, the Medicare and Medicaid chief, who recently became a regular presence at Mr. Trump’s televised briefings on the pandemic. Mr. Azar did not include Dr. Hahn on the virus task force he led, though some of the F.D.A. commissioner’s aides participated in H.H.S. meetings on the subject.
And tensions grew between the secretary and Dr. Redfield as the testing issue persisted. Mr. Azar and Dr. Redfield have been on the phone as often as a half-dozen times a day. But throughout February, as the C.D.C. test faltered, Mr. Azar became convinced that Dr. Redfield’s agency was providing him with inaccurate information about testing that the secretary repeated publicly, according to several administration officials.
In one instance, Mr. Azar appeared on Sunday morning news programs and said that more than 3,600 people had been tested for the virus. In fact, the real number was much smaller because many patients were tested multiple times, an error the C.D.C. had to correct in congressional testimony that week. One health department official said Mr. Azar was repeatedly assured that the C.D.C.’s test would be widely available within a week or 10 days, only to be given the same promise a week later.
Asked about criticism of his agency’s response to the pandemic, Dr. Redfield said: “I’m personally not focused on whether they’re pointing fingers here or there. We’re focused on doing all we can to get through this outbreak as quickly as possible and keep America safe.”
For all Mr. Azar’s complaints, however, he continued to defer to the scientists at the two agencies, according to several administration officials. Mr. Azar’s allies said he was told by Dr. Redfield and Dr. Fauci that the C.D.C. had the resources it needed, that there was no reason to believe the virus was spreading through the country from person to person and that it was important to test only people who met certain criteria.
But even in the face of a crescendo of complaints from doctors and health care researchers around the country, Mr. Azar failed to push those under him to do the one thing that could have helped: broader testing.
In a statement, Caitlin Oakley, Mr. Azar’s spokeswoman, said that the secretary had “empowered and followed the guidance of world-renowned U.S. scientists” on the testing issue. “Any insinuation that Secretary Azar did not respond with needed urgency to the response or testing efforts,” she said, “are just plain wrong and disproven by the facts.”
By Feb. 26, Dr. Fauci was concerned that the stalled testing had become an urgent issue that needed to be addressed. He called Brian Harrison, Mr. Azar’s chief of staff, and asked him to gather the group of officials overseeing screening efforts.
Around noon on Feb. 27, Dr. Hahn, Dr. Redfield and top aides from the F.D.A. and H.H.S. dialed in to a conference call. Mr. Harrison began with an ultimatum: No one leaves until we resolve the lag in testing. We don’t have answers and we need them, one senior administration official recalled him saying. Get it done.
By the end of the day, the group agreed that the F.D.A. should loosen regulations so that hospitals and independent labs could move forward quickly with their own tests.
But the evening before, Mr. Azar had been effectively removed as the leader of the task force when Mr. Trump abruptly put Mr. Pence in charge, a decision so last-minute that even the top health officials in the White House learned of it while watching the announcement.
A Tacit Acknowledgment
Previous presidents have moved quickly to confront disease threats from inside the White House by installing a “czar” to manage the effort.
During an outbreak of the Ebola virus in 2014, President Barack Obama tapped Ron Klain, his vice president’s former chief of staff, to direct the response from the West Wing. Mr. Obama later created an office of global health security inside the National Security Council to coordinate future crises.
“If you look historically in the United States when it is challenged with something like this — whether it’s H.I.V. crises, whether it’s pandemic, whether it’s whatever — man, they pull out all the stops across the system and they make it work,” said Dr. Aylward, the W.H.O. epidemiologist.
But faced with the coronavirus, Mr. Trump chose not to have the White House lead the planning until nearly two months after it began. Mr. Obama’s global health office had been disbanded a year earlier. And until Mr. Pence took charge, the task force lacked a single White House official with the power to compel action.
Since then, testing has ramped up quickly, with nearly 100 labs at hospitals and elsewhere performing it. On Friday, the health care giant Abbott said it had received emergency approval for a portable test that could detect the virus in five minutes.
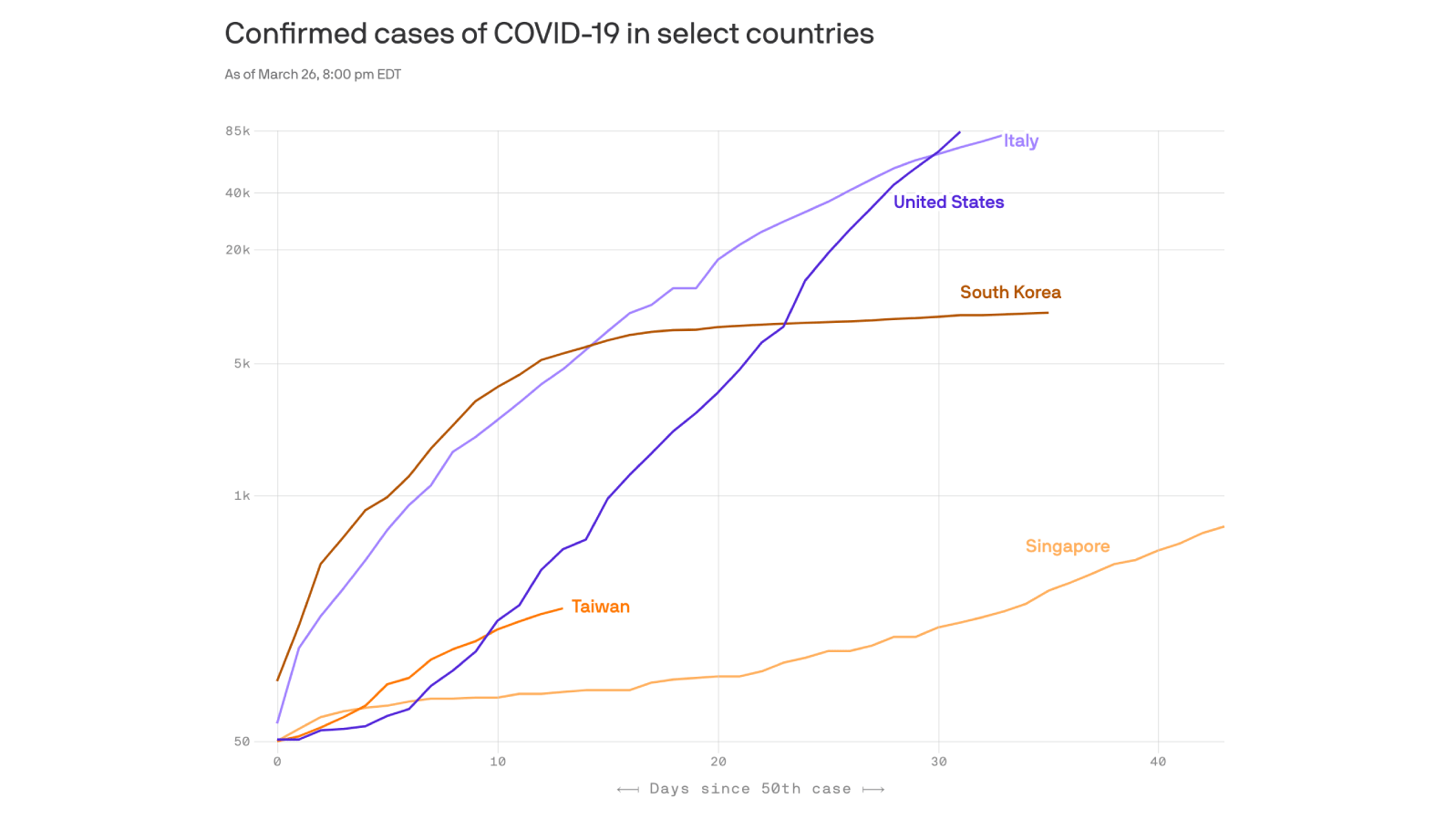
The president boasted on Tuesday that the United States had “created a new system that now we are doing unbelievably big numbers” of tests for the virus. The U.S., he said, had done more testing for the coronavirus in the last eight days than South Korea had done in eight weeks.
Yet hospitals and clinics across the country still must deny tests to those with milder symptoms, trying to save them for the most serious cases, and they often wait a week for results. In tacit acknowledgment of the shortage, Mr. Trump asked South Korea’s president on Monday to send as many test kits as possible from the 100,000 produced there daily, more than the country needs.
Public health experts reacted positively to the increased capacity. But having the ability to diagnose the disease three months after it was first disclosed by China does little to address why the United States was unable to do so sooner, when it might have helped reduce the toll of the pandemic.
“Testing is the crack that split apart the rest of the response, when it should have tied everything together,” said Dr. Nahid Bhadelia, the medical director of the Special Pathogens Unit at Boston University School of Medicine.
“It seeps into every other aspect of our response, touches all of us,” she said. “The delay of the testing has impacted the response across the board.”