American Health Insurance Diddy

there’s an office in a building
and a person in a chair
and you paid for em both
though you may be unaware
you paid for the paper
you paid for the phone
you paid for everything they need
to deny u wut yer owed
there aint no u in united health
there aint no me in the company
there aint no us in the private trust
there’s hardly humans in humanity
no the procedure that yer needing
aint the cost effective route
and only two percent of people end up winnin a dispute
so if u get sick
pray to god for help
cause yer doctor’s gotta pray thru
united health
waay back in 70 and 7
mr richard t burke
started buyin hmo’s
puttin federal grants to work
made 50 billion buckaroos
last yr
the warren buffet of health
the jeff bezos of fear
now ceo’s come and go
and one jus went
the ingredients ya got
bake the cake ya get
but if u get sick
cross yer fingers fer luck
cus ole richard t burke
aint givin a fuck
commoditized health
monopolized fraud
“here’s the doctors we own”
“here’s the research we bought”
they own the pharmacies
and alotta the meds
they should start buying graves
to sell us when we’re all dead
there aint no u in united health
there aint no me in the company
there aint no us in the private trust
there’s hardly humans in humanity
there’s hardly humans in humanity
New HHS Rule Wipes Out Some Public Comment on Rulemaking

A 3-page ruleopens in a new tab or window published in the Federal Register today and signed by HHS Secretary Robert F. Kennedy Jr. ends the ability of stakeholders to comment on many of the agency’s policies regarding benefits, contracts, and grants within the agency.
“The intent of this policy is very clearly to enable the administration to adopt major policy changes very quickly, without first letting the public know what those changes are going to be,” said Samuel Bagenstos, JD, who served as general counsel to the Office of Management Budget and subsequently HHS during the 4 years of the Biden administration.
Under this new policy, which says it “is rescinding the policy on public participation in rule making,” rules issued by any of the divisions within HHS that fall under the Administrative Procedure Act (APA) would be affected — except for Medicare, which falls under a separate provision of the Medicare Act, Bagenstos told MedPage Today during a phone call Friday.
Medicaid, the Substance Abuse and Mental Health Services Administration, the Administration for Children and Families, the National Institutes of Health, and many other agencies fall under this new rule, he said, for all policies having to do with grants or benefits or both.
The policy ends a practice that has been an important part of U.S. healthcare for more than 50 years.
“For example, if they wanted to allow work requirements under Medicaid, they could do that now … without going through rule changing policies,” said Bagenstos, who now is a professor of law at the University of Michigan in Ann Arbor.
Bagenstos said he doubts the new rule “is going to hold up in court. There are very substantial grounds to challenge this as being arbitrary and capricious.”
Typically, HHS issues a notice of proposed policies and then allows a period, typically 60 days, for interested and affected parties to give feedback on how the rule would impact them and/or the public. Often hundreds and sometimes thousands of comments in support or opposition are typically posted on regulations.govopens in a new tab or window for each proposed rule. After the comment period, the agency reviews each comment and often provides a written response in the final rule explaining why the provision was or wasn’t finalized.
This new rule contends that the APA exempts the agency from having to adhere to the commenting process in rulemaking when the matter relates to “agency management or personnel or to public property, loans, grants, benefits or contracts.”
In 1971, HHS adopted a policy that waived the APA’s statutory exemption from procedural rulemaking requirements, the so-called “Richardson Waiver.” The waiver required HHS to use notice and comment rulemaking procedures.
But under the new rule, that waiver is “contrary to the clear text of the APA and imposes on the Department obligations beyond the maximum procedural requirements specified in the APA.”
It concludes, “Effective immediately, the Richardson Waiver is rescinded and is no longer the policy of the Department.”
The new rule relieves these agencies of a tremendous amount of work. It states: “The extra-statutory obligations of the Richardson Waiver impose costs on the Department and the public, are contrary to the efficient operation of the Department, and impede the Department’s flexibility to adapt quickly to legal and policy mandates.”
Steven Balla, PhD, co-director of the George Washington Regulatory Studies Center in Washington, D.C., said that while it’s unclear how the new policy will be enforced, “It hit me out of the blue.”
“There’s historically been a bipartisan consensus that there are these two practices that you should follow when writing rules, and one is to seek public input, and the other is to do regular regulatory impact analysis. You have studies of the costs and benefits, the likely impacts of what you’re going to do,” he said.
He thinks that going forward, policies that must be published in the Federal Register “that have the full force of law as a regulation would all still have to go through notice and comment, unless the agency [invokes] a good cause exemption from the Administrative Procedure Act.”
The announcement also seems inconsistent with the Trump administration’s stated goal to improve transparency in public policy, a key element of which is public involvement that would be taken away, he said. “It’s a big deal, for sure.”
In the hours following the unpublished rule’s posting on Friday, several organizations expressed opposition mixed with confusion.
Stella Dantas, MD, president of the American College of Obstetricians and Gynecologists (ACOG), said in a statement that such a policy could weaken the healthcare system and harm patients and clinicians.
“The practice, delivery, and regulation of medicine is incredibly complex. The experiences of patients, clinicians, administrators, and other stakeholders across medicine must be taken into account in order to avoid unintended outcomes,” she said. Expert input from medical societies, researchers, and patient advocates is necessary “to inform regulatory bodies and ensure the soundness of final rules and other actions.”
Kate Smith Sloan, president and CEO of LeadingAge, an association of 5,400 non-profit organizations including nursing homes that provide a variety of services for seniors, echoed many of ACOG’s views. In a statement, she said the policy “has the potential to significantly harm older adults and the nonprofit providers who serve them.”
“The possibility that HHS under the Trump White House will eliminate or significantly scale back public comment on policies impacting payment, regulations, safety, operations, and other critical areas is truly troubling — a move we can only hope will not have the negative impact that we fear it might,” she said.
Ted Okon, MBA, executive director of the Community Oncology Alliance, a non-profit organization of oncology practices, told MedPage Today in an email that the administration needs to provide more clarification on the rule. But he said the ability to comment on any policy impacting cancer care “is critical … to provide agencies with real-world data and insight that is not available to them in D.C.”
Alice Bers, JD, litigation director for the Center for Medicare Advocacy, said that the “likely attempt to avoid public comment on actions and policies the agency expects will be unpopular” and “will have broad impact across HHS and its subagencies.”
Like Bagenstos, Bers doesn’t think the changes would impact Medicare policy, which has its own notice and comment requirements under the Medicare Act separate from the APA.
It was not immediately clear whether the HHS under Kennedy plans to pursue additional policy changes on annual Medicare rulemaking, a complex process that affects payment amounts, reporting, qualification and quality requirements affecting hospitals, physician practices, nursing homes, hospices, and many other healthcare settings.
Said Bagenstos: “They’d need to get Congress to repeal it [which] I can’t really see happening.”
Several large healthcare advocacy organizations appeared caught off guard by the new rule.
Representatives of the American Medical Association, the American Hospital Association, and the California Hospital Association said on Friday they were reviewing the new policy.
Cartoon – Leadership Today
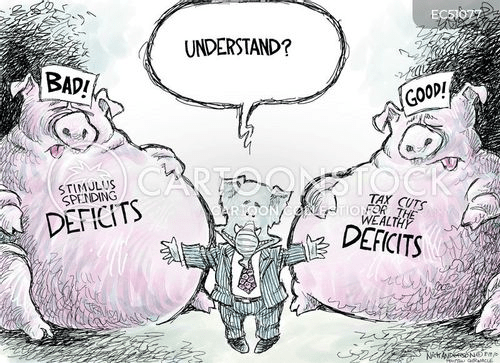
Cartoon – Deficit Expansion
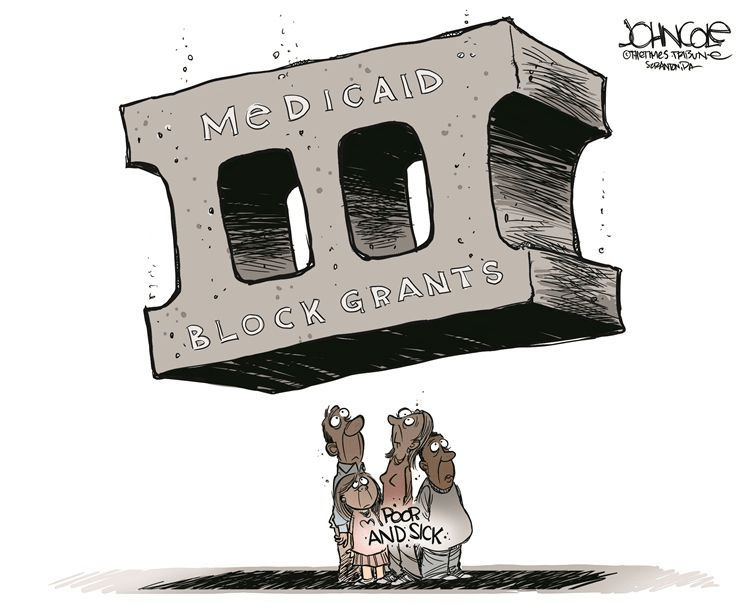
Cartoon – Medicaid Block Grants
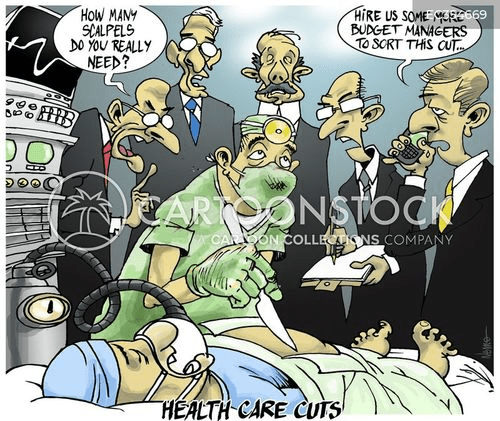
Cartoon – Health Care Cuts
Cartoon – Medicare Advantage Office Visit
Cartoon – Inexpensive Medical Insurance
Key Principles for Proactive Management of Patient Denials
https://www.kaufmanhall.com/insights/article/key-principles-proactive-management-patient-denials

The proliferation of claims denials, especially by Medicare Advantage payers, has become a pressing issue for health system operations. In 2023, Medicare Advantage insurers fully or partially denied 3.2 million prior authorization requests—or 6.4% of all requests, according to a Kaiser Family Foundation (KFF) report.
The growth in denials can be partially explained by the increasing popularity of managed Medicare and Medicaid plans, but evolving payer practices, including the adoption of AI for algorithmic denials, have also contributed. Claims denials have emerged as one of the key points of payer-provider tension, and an effective claims denials management and prevention program is a powerful way for health systems to rebalance their payer relationships.
Denied claims result in reduced reimbursement, added administrative burdens, and patient and provider frustrations. Even when denials are successfully appealed and reversed—the KFF report found that in 2023, 82% of Medicare Advantage denials were partially or fully overturned—the time and resources devoted to the appeals process add to the costs of providing healthcare services. Optimizing pre-billing activities to reduce avoidable denials and improve and streamline the patient experience of care is as essential for health systems as a robust appeals strategy. This article addresses critical success factors for both preventing and appealing denials.
Preventing Claims Denials During Pre-Bill Period
Successfully preventing denials requires a centralized program across the workforce, from frontline providers to clinical and revenue cycle staff, to manage pre-bill activities by focusing on identifying the correct patient insurance information, obtaining accurate authorizations, and preventing concurrent denials while the patient is still in the facility. Utilization review nurses, attending providers, and Physician Advisors should be attentive to documenting the full state of patient acuity, while collaborating with the revenue cycle team. This team should focus on the collection and reporting of medically necessary data and documentation, which serves as the evidence payers use to evaluate prior authorization requests. When information about a patient’s condition isn’t recorded, or acknowledged in an authorization request, unnecessary denials can result.
A successful denials prevention program expands beyond the utilization management (UM) team and includes revenue cycle, and provider collaboration. Revenue cycle pre-service procedures should focus on confirming insurance benefits and securing payer authorization for planned services while collaborating with UM and referral sources. A comprehensive and proactive denials prevention program helps conveys to payers the full extent of inpatient clinical work, thanks to a collaborative effort to improve documentation.
The following list can help organize denials prevention programs across all locations, clinics and practices:
- Establish an enterprise-wide denials prevention strategy which includes a multi-disciplinary denials management committee focused on identifying denials trends, conducting root cause analyses, developing proactive denials mitigation plans, creating enhanced reporting, monitoring improvement, and communicating risk
- Establish proactive revenue cycle, UM, pre-certification, and peer-to peer workflows procedures to confirm completion of payer requirements prior to scheduled services and discharge
- Ensure patients are financially cleared through implementation of pre-service protocols, including enhanced medical necessity process for outpatient services, authorization defer and delay procedures to reduce rework and avoidable denials
- Identify pre-bill edits to increase “clean claim” efficiency, reducing initial denials and expediting reimbursement
- Deliver education to providers, care management, and nursing teams on key observation concepts, such as clinical documentation improvement, patient status documentation, medical necessity documentation and orders for the Two Midnights rule, and payer reimbursement methodologies
Pursuing Post-Bill Appeals, Reversals and Payer Escalation
A strong denials management and prevention program should include a robust post-bill appeals program with skilled coding, clinical and technical resources. A targeted and strategic appeal process can result in improved overturn rates and increased reimbursement. Appeal letters which are supported by clinical facts, payer policies, and a summary of key components relevant to each case and the associated denial increase the likelihood of success.
Components of the appeal program should include the following:
- Guidelines for when to appeal based on potential success by payer and appeal level
- Reviews of upheld appeals for second and third level appeals based on strategy by payer
- Trends for all upheld appeals by reason and by payer
- Dashboard for tracking denials activities
- Appeal letter writing guidelines and tips to support
- Evaluation process for existing payer escalation workflows, tools and payer communication strategies with consideration for payer
- Process to measure and monitor overturn rates and improvement opportunities
The collaboration with managed care is vital to the success of the denials management/prevention program. A formal payer escalation process which facilitates transparency between the payer and provider can result in improved relations and a reduction in initial denials. Successful denials management/prevention payer escalation programs are strategic and focus on addressing unfair/incorrect denials and establishing clear bi-directional reporting and communications. These programs can result in improved contract negotiations and reduce incorrect denials.
Artificial Intelligence (AI) can support the post-bill appeals process and can be especially relevant when developing a strategy to combat denials. Not only are payers increasingly using AI to trigger denials, but health systems can also deploy AI to write appeal letters, analyze denial trends, and summarize medically necessary documentation. Although algorithmic denials have become a source of frustration for providers and patients, health systems can also deploy AI to their defense. While payers are often better positioned to devote AI resources to claims, a little bit of investment from health systems, deployed effectively, can go a long way toward evening the playing field.
Closing Thoughts and Seven Questions to Consider
A formal denials management and prevention program is essential to obtaining proper reimbursement for the care provided and reducing rework across the enterprise. A strong program should also improve the patient’s experience of care: ideally, a patient should not need to interact with or hear from their provider between scheduling an appointment and checking in.
Denials management and prevention programs should be led by multi-disciplinary committees and focus on reducing avoidable denials and rework. Reducing denials requires the implementation of a multi-disciplinary program and collaboration between UM, revenue cycle, clinical documentation improvement, managed care, clinical operation and providers.
Health systems reassessing their claims denials program should consider these questions:
- Do you have a reactive or proactive denials management strategy in place?
- Does your denials strategy include multi-disciplinary team representation?
- What reporting/tools are currently being used to track and manage denials?
- What are your top five denial categories and what is being done to address the root cause of these denials?
- How are avoidable denial risks managed, communicated and monitored?
- Have you implemented a comprehensive denials management strategy with a multi-disciplinary committee?
- Are the system’s internal resources and expertise sufficient for addressing identified challenges, or should the system seek external partners to implement changes?