At least 11,357,000 cases have been reported.
The disease caused by the novel coronavirus has killed at least 247,000 people in the United States since February and has enveloped nearly every part of the country.
As health officials long predicted, autumn brought soaring case counts, strained hospital capacity and increased deaths nationwide, as the virus is not only popping up in new places but also circling back to areas that once appeared to have it contained. Nearly all metrics in most of the country are trending in the wrong direction.
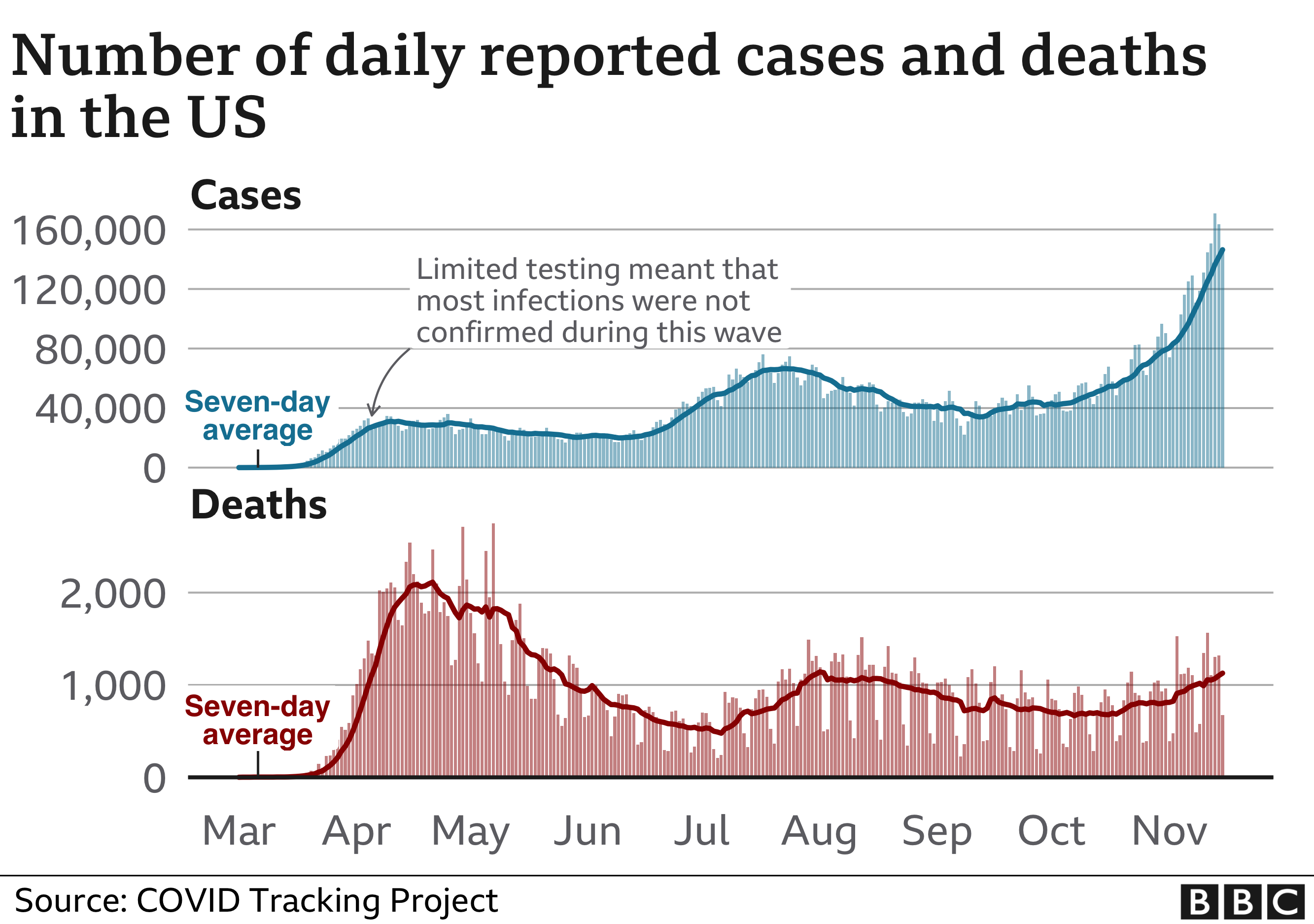
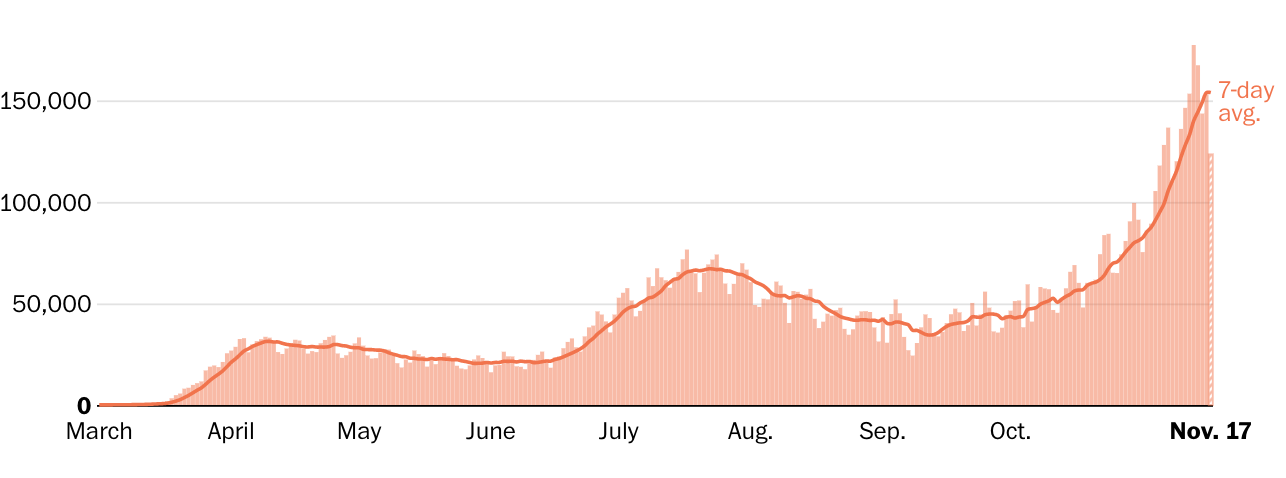
During an April peak, the seven-day-average U.S. death toll hit more than 2,000 per day, but cases were concentrated largely in the Northeast. During a July lull, average deaths sank to a low of 463 per day, although cases surged in the Sun Belt.
By early November, however, the country was recording more new cases than ever — well over 100,000 per day — and many states reported record-high caseloads and hospitalizations. The average U.S. deaths per day again shot past 1,000, despite improvements in treatment that make survival more likely.
In the past week in the U.S….New daily reported cases rose 26% New daily reported deaths rose 13.5% Covid related hospitalizations rose 23.9%.
Among reported tests, the positivity rate was 10%.
The number of tests reported rose 9.6% from the previous week.
Numbers in this article have fluctuated as testing and reporting criteria have evolved, particularly in areas that were hit early. Three spikes in the deaths chart above reflect large, one-time adjustments: In mid-April, New York City added more than 3,700 deaths. New Jersey added more than 1,800 on June 25. And in September, The Post changed its methodology for reporting deaths in New York and added a one-day increase of more than 2,700 on Sept. 18.
Health officials, including the country’s top infectious disease expert, Anthony S. Fauci, have said the virus has killed more people than official death tolls indicate.
No longer concentrated solely in a few urban areas or in nursing homes, prisons and factories, the virus seems to flourish wherever people let down their guard.
New York, which was slammed with the new disease in spring and where at least 33,000 have died, is one of several states experiencing a second or even third wave.
Sun Belt states had serious outbreaks after Memorial Day when people flocked to beaches. By late summer, parts of the Midwest were inundated. In August and September, clusters appeared in newly reopened college campuses, particularly in the Northeast and Midwest. By October, Upper Midwest, Great Plains and Western states that had previously been mostly spared were reporting major outbreaks, including Minnesota, Wisconsin, Arkansas, the Dakotas and Alaska.
In November, most states reported record-high case counts and greater demand for hospital beds. Several set records for single-day fatalities.
In the absence of a federal plan, containment strategies vary by state and locality and have often reflected political polarization. The mounting crush of cases this fall, however, has prompted officials of both parties to tighten mask mandates, reimpose restrictions on gatherings and discourage holiday travel and gatherings to try to squelch the spread.
A majority of states and many retail chains required masks in public places by late July, and public health officials touted them as one of the easiest ways to stop the pandemic. Still, some people in even the hardest-hit areas refuse to wear them, despite evidence that they protect wearers and those around them.
People older than 65 and those with obesity and underlying health problems are the mostly likely to die from covid-19, but a large percentage of infections occur in younger, more mobile people. People younger than 40 tend to become less sick but also unknowingly may pass the disease to others around them.
The virus rarely kills children, although researchers have linked it to a mysterious and deadly inflammatory syndrome.
Outbreaks of covid-19 have hit Black, Hispanic and Native American communities particularly hard.
Sparsely populated areas don’t have the huge raw numbers that cities have reported, but some rank among the highest in deaths and cases per capita.
By late October, covid-19 had been documented in all but three U.S. counties and areas in Montana, the Dakotas and Idaho had some of the highest per capita caseloads.
People in very rural areas may be more vulnerable to covid-19 than urbanites, according to a Post analysis of CDC data.
Testing was slow to begin in the United States, and a system has yet to be standardized.
Demand has often overwhelmed testing infrastructure, muddying the ability of officials to get a true picture of the virus’s reach. In June, CDC Director Robert Redfield estimated that, based on antibody tests, the actual number of U.S. residents who had been infected by the virus was likely to be 10 times as high as the number of confirmed cases. More recently, conflicting CDC guidelines about whether people without symptoms should be tested caused confusion and inhibited contact tracing.
A sharp increase in hospitalizations in late October and November demonstrates that the virus is spreading, not just that more testing is finding more asymptomatic cases. A group of Illinois health-care workers predicted in a Nov. 10 open letter to state and Chicago officials that “Illinois will surpass its ICU bed capacity by Thanksgiving.”
Some hospitals, straining to find beds and health-care workers to handle the crush of patients, are considering unusual measures.
In North Dakota, health-care workers who test positive but have no symptoms can continue working in covid-19 wards, according to Gov. Doug Burgum (R). Some facilities in Oklahoma, Kansas and Ohio are limiting routine care and deferring non-emergency surgeries.
Not all news is bleak, however.
On Nov. 9, Pfizer announced that its promising vaccine — one of many in the works — appeared more than 90 percent effective in an ongoing trial. The same day, regulators granted emergency authorization to an antibody treatment that may keep mild illness from becoming severe.
And the next day, Fauci told CNN that the average American may have access to a vaccine by April.